Improving fuel cell efficiency through a deep understanding of electrochemistry
Bosch Research Blog | Post by Ulrich Berner and colleagues, 2021-01-14

Co-authors: Christophe Gerling, Matthias Hanauer
The heart of every fuel cell is a thin membrane covered with a catalyst layer on both sides. Although it looks rather unspectacular, this humble membrane is where the magic of the fuel cell takes place. The basic reactions in this crucial component are well known. However, a complete understanding of the complex processes is needed to improve the efficiency of the fuel cell and get fuel cell electric vehicles on the road.
Vision: fuel cells for dedicated applications
Working towards carbon neutrality requires drastic changes in how we fuel our transport. However, in developing new fuel technologies, the cost, weight, lifetime, and peak performance must be carefully balanced. We at Bosch think that the fuel cell is a crucial solution in overland transport, especially in applications with heavy loads and long distances. To make this vision a reality, we are entering the market of mobile fuel cells in trucks and cars.
Challenge: efficiency loss
To reach carbon neutrality, we are combining Bosch’s long-lasting expertise in system integration competence with our state-of-the-art fuel cell stack technologies. However, although fuel cell technology has been proven viable and robust, several obstacles hinder attempts at large-scale replacement of the combustion engine. One major challenge lies within the fundamental process of every fuel cell: the chemical reaction converting hydrogen and oxygen into electricity and water. This reaction is responsible for most of the efficiency loss in the form of waste heat.

To improve the efficiency of fuel cells, an in-depth understanding of the electrochemistry is mandatory.
The basic aspects of the electrochemical reaction which takes place in the catalyst layer have been known for a long time. However, in order to improve overall efficiency, we need a complete understanding of all processes occurring in the catalyst layer under all relevant conditions. To do this, we must separate the effects of the chemical reaction from other concurrent processes (including electrical/proton conduction, mass transfer, etc.). Thus, we at Bosch Research are working with university partners on sophisticated characterization methods and new simulation models to further improve our understanding of all processes during fuel cell operation.
Approaching the solution with reaction kinetics
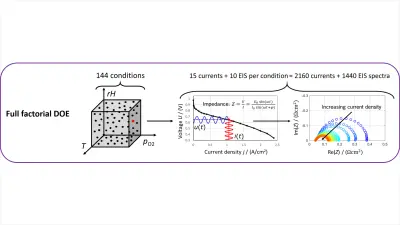
One activity to elucidate details of the electrochemical reaction involves the research of our PhD student, Christophe Gerling, who investigated the reaction kinetics under an unprecedentedly large set of operation parameters. (Research paper currently in review, for more information see also PEMFC Model Parameterization By Means of Differential Cell Polarization and Electrochemical Impedance Spectroscopy.) By using a number of characterization tools, including Electrochemical Impedance Spectroscopy (EIS), Distribution of Relaxation Time (DRT) and Limiting Current measurements, we could delineate the impact of the chemical reaction and all the other processes occurring in parallel.
This allowed us to parametrize the kinetics of the reaction over a large range of relevant operation conditions and revealed interesting interactions and operation optima. For instance, we found that the humidity of the gases influences several of the processes in different ways. While some components of the catalyst layer function best under full humidity, overall performance could also be optimized to lower humidity levels, possibly lowering system cost. This might help in finding improved humidification strategies.
Outlook
In the future, we plan to use this knowledge to derive design rules for the catalyst layer itself. This can help improve the performance of the layer as well as lowering the need for expensive catalyst materials.
This allowed us to parametrize the kinetics of the reaction over a large range of relevant operation conditions and revealed interesting interactions and operation optima. For instance, we found that the humidity of the gases influences several of the processes in different ways. While some components of the catalyst layer function best under full humidity, overall performance could also be optimized to lower humidity levels, possibly lowering system cost. This might help in finding improved humidification strategies.
What are your thoughts on this topic?
Please feel free to share them via LinkedIn or to contact me directly.
Author: Ulrich Berner
Ulrich is a chemical engineer by training and has worked in multiple areas relating to energy conversion and storage. After his PhD on the topic of thin film photovoltaics, he started at Bosch Research on a project dealing with PEM electrolyzers and electrochemical compression. After a 2-year intermission working on solid state lithium polymer batteries, Ulrich returned to the PEM world with a focus on cause-effect relationships in the performance and degradation of fuel cell electrodes.

Co-authors: Christophe Gerling, Matthias Hanauer


