New trend in healthcare: small yet powerful
Bosch Research Blog | Post by Young Shik Shin, 2021-04-07

When I started my journey as a biomedical engineer about 18 years ago, my advisor handed me over an article that introduced new concepts such as lab-on-a-chip, bio-MEMS (micro-electromechanical system) and microfluidics. A common feature of these technologies is miniaturization and integration of multiple functional elements by actively utilizing microfabrication technologies (which, by the way, is one of the core competences of Bosch). A dream goal that the article tried to envision through those technologies was a future of medicine that offers disease diagnostics with a single drop of blood in a short time period. After a little less than two decades, this dream goal is no longer just a dream.
Healthcare in an era of change
Numerous inventions and innovations have brought us medical test equipment that requires only small amounts of blood that you can obtain with a quick prick of the finger. In fact, from the research point of view the Vivalytic platform from Bosch Healthcare Solutions can also run a PCR test with just 50 microliters of sample volume (literally a single drop of blood). Miniaturization not only allows us to integrate multiple functions into a small device but also accelerates biochemical reactions. The recent Vivalytic rapid PCR SARS-CoV-2 test with the Vivalytic system takes less than 39 minutes, thanks to a fully automated process with a disposable cartridge. We are now living in an era of rapid transitioning in healthcare practice: fast, accurate and more personal, all owing to small yet powerful technologies. Exciting, isn’t it? But what’s next?

Microenvironment: localized and temporal control
We are talking about miniaturization, but you can always think about an even smaller fraction of an already small volume. In biology, microenvironment draws special interest these days, especially in cancer research. Cancer cells are very aggressive, yet smart, and know how to change the local environment around them for their own benefit. They change the local concentration of certain proteins that affect neighboring cells or even recruit specific types of cells toward them. As you might know, cells are tiny, about 10 micrometers in diameter, which is roughly one tenth of the diameter of your hair. Therefore, the volume that we are talking about here is extremely small. The volume of a single cell is in picoliter range: 1/1,000,000 of a microliter. Even though it is a tiny volume, people are learning more and more interesting facts from single cells that are clinically relevant.
We can also extend this toward a slightly larger number of cells, about 100 to 1,000 cells, which is still in nanoliter range (1/1,000 of a microliter). Adoptive immunotherapy, for example, is a very promising cancer treatment option. One major process of this therapy is to harvest the patient’s own T-cells, grow them (increase their number) in the laboratory, and infuse them back to the patient. Another interesting approach is the organ-on-chip. Many research groups and companies are trying to develop technologies that mimic functions of a patient’s organ in a micro-device with the patient’s own cells.
- is the diameter of a cell, which is roughly one tenth of the diameter of your hair
- of 1 microliter is the volume of a single cell
All these approaches require miniaturized devices with a unique design to realize specific functions. Currently available devices have relatively simple designs that can provide a positive environment for the cells and perform basic analyses, mostly based on optics. Cells are, however, very dynamic and sensitive to the environment. Thus, the next question to ask is if we can control some of the core environmental factors more precisely within a small volume of liquid sample. It would be even more powerful if we could control the microenvironment on-demand with temporal changes. For example, we could envision the following scenario: capturing specific cancer cells from the circulating blood, growing them in a micro device, treating them with a drug at a given point in time, monitoring their reaction and harvesting them for further downstream analysis. It sounds ambitious, but many research groups worldwide are actively working on a suite of technologies for such applications.
-

Together with his colleagues at the Bosch Research and Technology Center North America, Young Shik is working on active control of the microenvironment in a micro device.
On-demand pH modulation
To this end, my colleagues and I at the bioelectronics group at Bosch Research Technology Center North America have developed a technology that can generate microenvironments in which the pH value can be electrochemically controlled. We chose pH as a controlling parameter because it is an important property of aqueous solutions that plays a critical role in many chemical, biochemical and physiological systems. Current laboratory practice, when a change in pH value is needed, requires replacing the whole buffer solution or titrating with acid or base, which is a time consuming and tedious process. In contrast, the electrochemical approach that we developed allows changing the pH by simply applying current to an electrode in a solution.

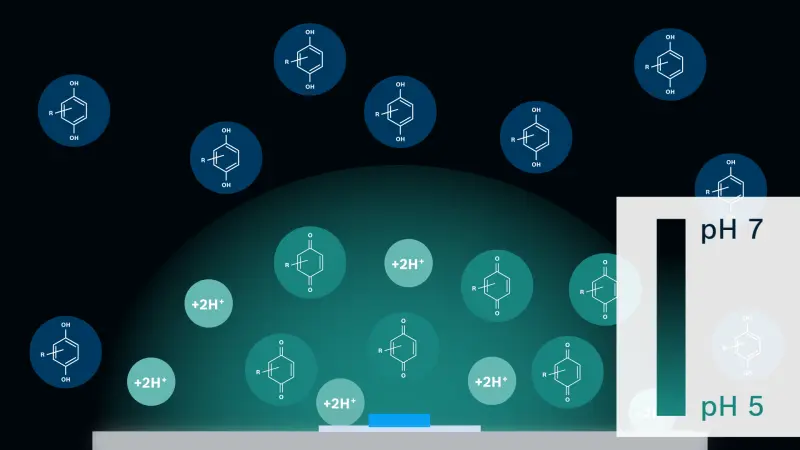
Our device has the same form factor as a commonly used microscope slide. The slide has two types of electrodes: one for sensing pH (sensing electrode, SE) and the other for modulating the pH microenvironment (working electrode, WE). We also add electroactive agents (quinones in our application) to the solution.
Learn more about the electrochemical platform for localized pH control on demand.
This is how it works: pH SE continuously monitors the pH of the solution close to the surface. Electrical current, chosen by the difference between the target pH and the actual pH measured by SE, is applied to the WE. Then, electroactive reagents – located close to the WE surface –undergo a proton-coupled electron transfer, which produces or consumes protons depending on the polarity of the current applied. Anodic current to the WE liberates protons, while cathodic current consumes protons. For the anodic current case, the liberated protons diffuse away from the surface and are neutralized by buffer molecules, which results in a pH modulation zone being confined in close proximity to the WE surface. Thus, the balance between the applied current and the buffer capacity determines the spatial extent of the pH modulation zone as well as pH. The opposite reaction happens for the cathodic current case.
It sounds complicated but the actual process is very simple. You can just set the parameters as needed (pH levels and time) and hit the ‘run’ button. The core value of this technology is that you can change pH locally (near the WE surface) without affecting the bulk buffer solution. As mentioned earlier, we have micro/nano fabrication technologies readily available, which means we can easily design a device that can generate many active pH modulation microenvironments that are independently controllable. The number can go up to hundreds, thousands or even millions. Considering the broad range of biochemical processes that are fundamentally affected by pH, including antibody-antigen interaction, protein aggregation/misfolding, enzymatic process control, controlled chemical release, and so on, this kind of technology can have quite an impact on micro medical devices and their applications in the near future.
Still a challenge: electrification
Even though the technology has a great potential, there are still challenges. One major challenge is electrification. So far, most technologies for life science applications have been using optics-based technologies such as fluorescence, absorbance, or colorimetry. Since the healthcare community, including academia and clinics, is very conservative, it may take strong efforts to introduce new methods and practices. However, there is an increasing number of recent research activities on electrification in diagnostics and medicine, ranging from wearable biosensor to neurological applications. As these efforts gain momentum, more and more opportunities will present themselves to actively utilize what we are currently using for consumer electronics or the automobile sector: tiny electrical devices with sensors and actuators (core Bosch competences). In the near future, we will see more solutions in healthcare sector, smaller yet more powerful.
Recent research on electrification in diagnostics and medicine
What are your thoughts on this topic?
Please feel free to share them via LinkedIn or to contact me directly.
Author: Young Shik Shin
Young Shik is an engineer with a broad background from mechanical engineering to medical diagnostics. Currently, he is a senior expert in the bioelectronics team at Bosch Research. His team is developing new technologies for next-generation medical diagnostics, especially for point-of-care applications.